Name an Element Whose Atoms Occur in Pairs
Triple Covalent Bonds Between Different Atoms. When such an attachment is formed bond dissociation energy is released and the process is 2.
Chapter 3 Ionic Bonding and Simple Ionic Compounds discussed ionic bonding which results from the transfer of electrons among atoms or groups of atoms.
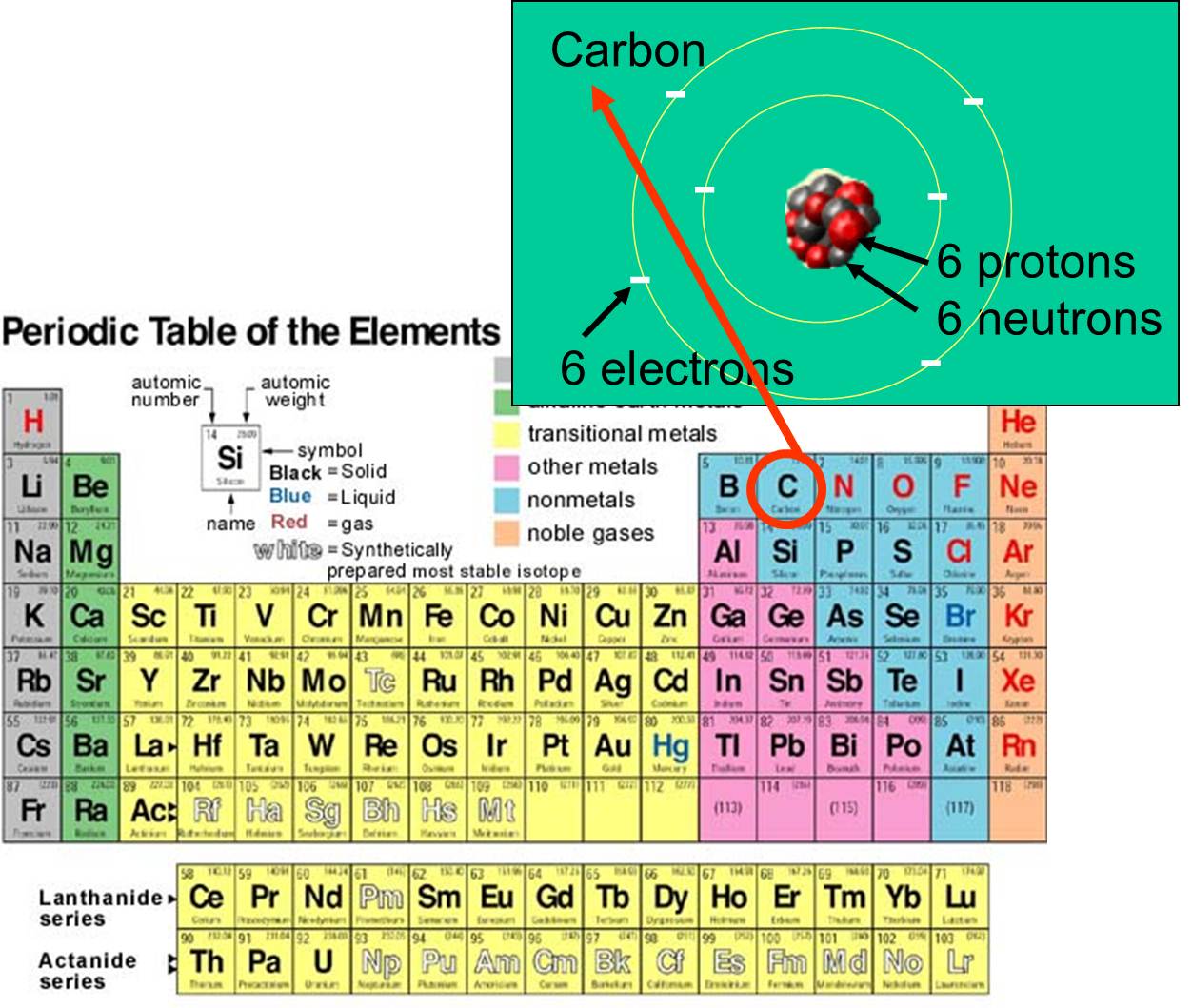
. Hydrogen and oxygen are 2 element whose atoms occur in pair in nature. B Compounds are composed of atoms of more than one element. A notation that shows what elements a compound contains the ratio of the atoms or ions of these elements.
Hydrogen is the only element whose isotopes have unique names. Anwesha Nayak added an answer on 8515. A solid whose atoms ions or molecules are arranged in a definite pattern.
A sugar whose molecules contain 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms f. These elements can exist in pure form in other arrangements. Some molecules in which three pairs of electrons are shared by two atoms contain triple bonds.
Name the family of elements that make 2 cations as shown in Model 2. For example oxygen can exist as the triatomic molecule ozone. Name any 2 element whose atoms occur in pair in nature - Science - Acids Bases and Salts.
A Elements are composed of atoms. 411 IONIC AND COVALENT COMPOUNDS AND THEIR. The atoms in a compound are combined through different types of bonds such as ionic covalent hydrogen and metallic bonding.
You can think of the atomic number as a kind of serial number of an element commencing at 1 for hydrogen and increasing by one for each successive element. Reactive elements have atoms that can combine to form compounds. D All atoms of a given element are identical.
1 There are total 7 diatomic elements 2 The elements which are not founded as the single atoms but only pairs of atoms is called the diatomic elements. Click again to see term. Hydrogen and oxygen are 2 element whose atoms occur in pair in nature.
Three isotopes of oxygen occur in nature. If two pairs of electrons are shared it is a ____ _____ and if three pairs are. In Model 2 there are several elements whose atoms make more than one type of.
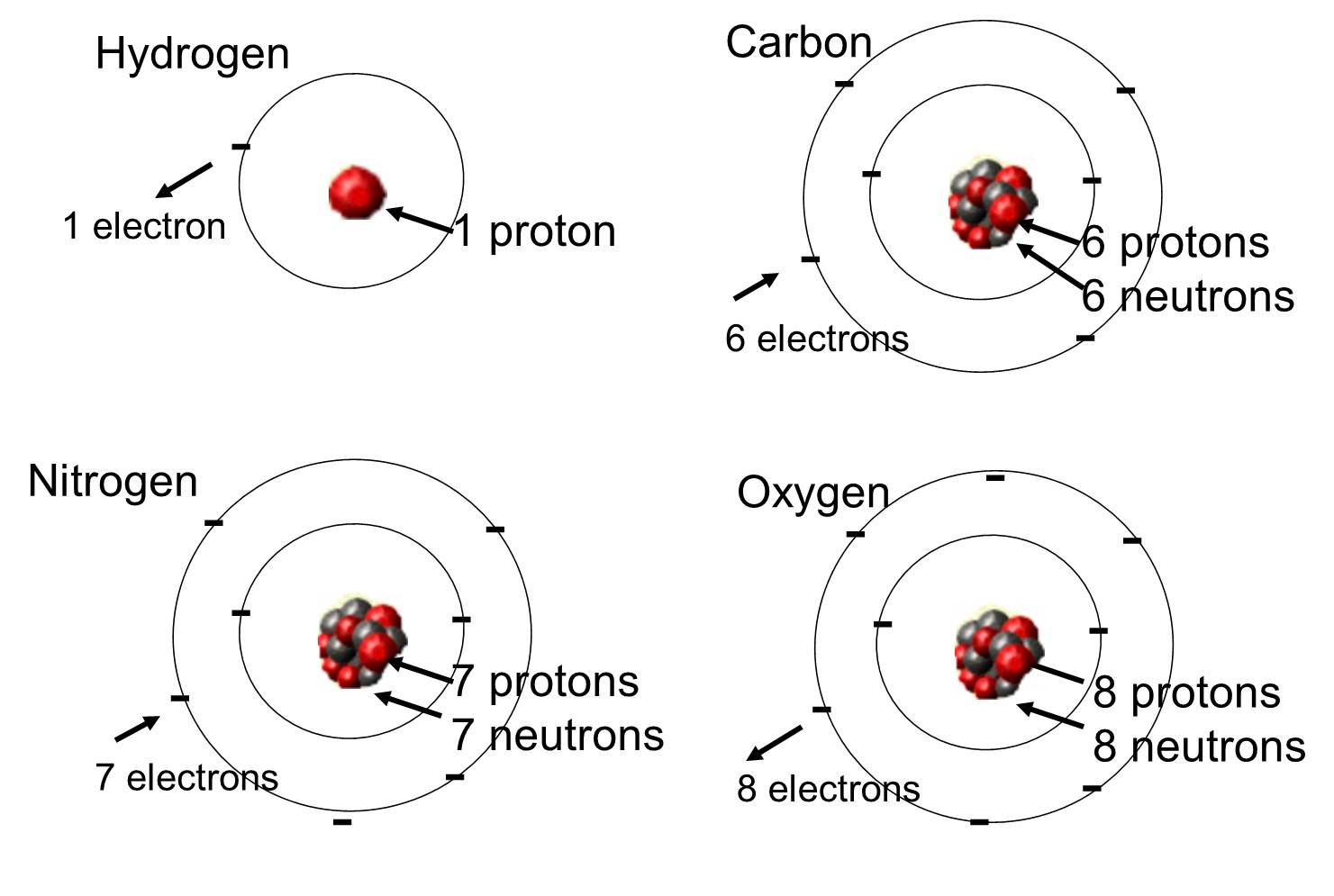
Atoms of the same element whose nuclei contain the same number of protons but different numbers of neutrons are called _____. A substance that contains the element whose atoms have 12 electrons indicate which element. Name the family of elements that make 1 anions as shown in Model 2.
Hydrogen nitrogen oxygen fluorine chlorine iodine bromine. Element Z has two naturally occurring isotopes. One shared pair of electrons between any two atoms is called a covalent bond.
Thus the symbol Sr stands for strontium whose atoms all have Z 38. The chemical name of the element and its symbol are uniquely tied to the atomic number. Oxygen is a diatomic element made up of 2 atoms of oxygen ie its atoms occur in pairs.
E The atoms of one element are the same as atoms of another element. Magnesium atoms have 12 electrons. Click again to see term.
Deuterium for hydrogen with one neutron and tritium for hydrogen with two neutrons. Neutrons exist to stabilize the nucleus without them the nucleus would consist of nothing but positively-charged protons in close proximity to one another. Anwesha Nayak answered this.
Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Name the major INORGANIC compounds in the body. What is name of change that occurs when atoms bond and electrons.
A compound containing aluminum atoms and also three times as many chlorine atoms as there are aluminum atoms e. Nitrogen belongs to group rmVleft rmA right of the periodic table and has 5 electrons in its outermost shell. Oxygen-15 oxygen-16 and oxygen-17.
A triple bond consists of the sharing of three pairs of electrons by two atoms. When sharing of electrons occurs the attachment between atoms that results is called a n 1 Click card to see definition. There are seven diatomic elements.
View Full Answer. Variation in the number of neutrons. Was this answer helpful.
Is a chemical bond in which two atoms share a pair of valence electrons. C A chemical reaction involves the rearrangement of atoms. Oxygen O 2 is an element whose atoms occur in pairs.
You have learned that not all atoms of an element are the same. When elements are not found as single atoms but only as a pair of atoms they are referred to as Diatomic elements. If the atomic mass of oxygen is 1599994 which of the three isotopes if any is most abundant.
If a pair of electrons is unequally shared between two atoms a _____ occurs. In this chapter we will consider another type of bondingcovalent bonding. Kaypeeoh72z and 3 more users found this answer helpful.
Why do atoms have different isotopes. A compound that contains twice as many potassium atoms as carbon atoms and three times as many oxygen atoms as carbon atoms. This manner of representing the hydrogen molecule HH is called its Lewis structure.
Tap again to see term. We will examine how atoms share electrons to form these bonds and we will begin to explore how the resulting compounds such as cholesterol. A double bond consists of the sharing of two pairs of electrons by two atoms.
Tap card to see definition. A simple compound that has a triple bond is nitrogen.
Understanding Atoms Elements And Compounds Lesson And Worksheets

No comments for "Name an Element Whose Atoms Occur in Pairs"
Post a Comment